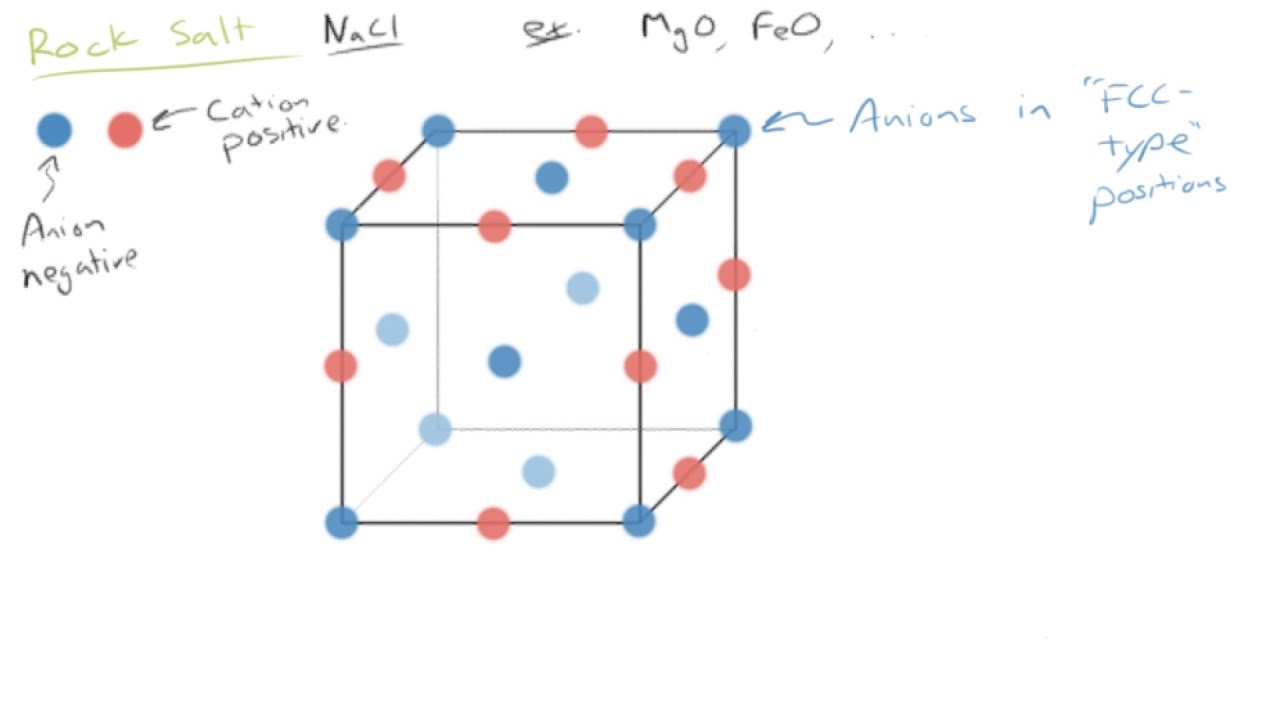
In ionically bonded ceramics some planes such as the so called 111 plane shown slicing diagonally through the rock salt structure in figure 3 top contain only one kind of ion and are therefore unbalanced in their distribution of charges.
Type of bonds that hold ceramic crystal structure.
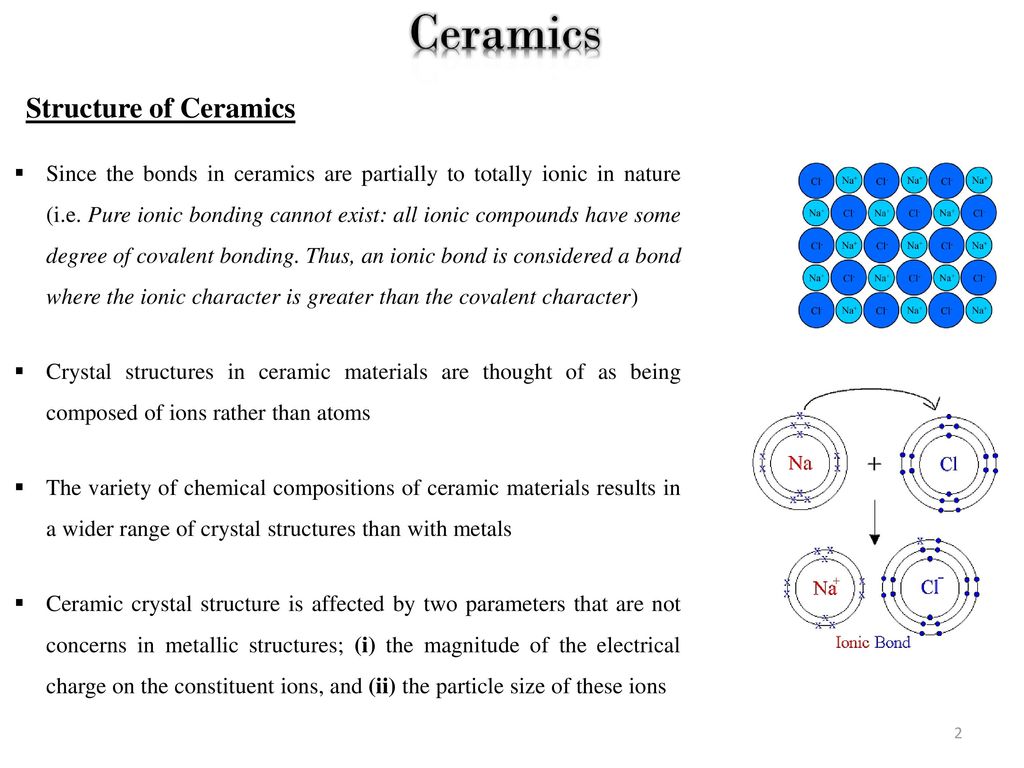
The ionic bond occurs between a metal and a nonmetal in other words two elements with very different electronegativity.
Underlying many of the properties found in ceramics are the strong primary bonds that hold the atoms together and form the ceramic material.
Crystal structures ceramic structure ax type crystal structures amxp type ambnxp type silicate ceramics carbon ceramic structures two or more different elements more complex than metal structures ionic and or covalent bonds a mix of ionic and covalent bonds electronegativity ionic bonds form ions.
The primary difference between ceramics and other materials is the chemical bonds that hold these materials together.
In ceramic composition and properties.
Ionic covalent metallic and molecular.
Electronegativity is the capability of the nucleus in an atom to attract and retain all the electrons within the atom itself and depends on the number of electrons and the distance of the electrons in the outer shells from the nucleus.
Although they can contain covalent bonds such as the si o si linkages in glass they are often characterized by ionic bonds between positive and negative ions.
The properties of a solid can usually be predicted from the valence and bonding preferences of its constituent atoms.
High hardness high compressive strength and chemical inertness.
Hydrogen bonded solids such as ice make up another category that is important in a few crystals.
If stress were applied to this structure it might shear along a plane where the bonds were weakest and read more.
When they form crystals the strong force of attraction between ions of opposite charge in the planes of ions make it difficult for one plane to slip past another.
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula al 2 o 3 it is the most commonly occurring of several aluminium oxides and specifically identified as aluminium iii oxide it is commonly called alumina and may also be called aloxide aloxite or alundum depending on particular forms or applications.
This is why ceramics generally have the following properties.
The two most common chemical bonds for ceramic materials are covalent and ionic.
These chemical bonds are of two types.
Two types of bonds are found in ceramics.
It occurs naturally in its crystalline polymorphic.
The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic.
Crystal crystal types of bonds.
Four main bonding types are discussed here.
The reasons for this lie in the nature of the bonds holding the crystal structure together.
They are either ionic in character involving a transfer of bonding electrons from electropositive atoms to electronegative atoms or they are covalent in character involving orbital sharing of electrons between the constituent atoms or ions.